

|
||
CHEMISTRY : Chromatography
Sample Analysis INK-002:
- Digital Edge Permanent Marker -
| PROJECT CODE: | 04.102 |
| SECTION: | CHROMATOGRAPHY |
| PROJECT TITLE: | Analysis on Ink Sample 002 (Digital Edge Permanent Marker) |
| RELEASE DATE: | 7 Sep 2009 |
| LAST UPDATE: | 20 Sep 2009 |
| VERSION HISTORY: | 1.0 - First release |
| Source | Permanent Marker |
|---|---|
| Sample Description or Commercial Name |
Digital Edge Permanent Marker |
| Sample Colour | Black |
| Solvents Tested | Methanol (50%) Ethanol (30%, 50%, 60%, 70%) Propanone (40%, 50%, 60%, 70%, 80%) Methanoic Acid (50%, 75%) Chloroform Chloroform/Kerosene mixture 1:1 Kerosene Formaldehyde Xylene DiMethylSulphOxide (50%, 80%) Propanone/Ammonia mixture (7:3) Propanone / Ethanol / Water mixture (1:1:2, 3:3:4) |
 |
|
| Rank | Solvent Name | No of Separated Dyes | Zones formation | Pattern Description | Picture |
|---|---|---|---|---|---|
| 1 | Methanol 50% | None | U | n/a | no |
| 2 | Ethanol 30% | None | U | n/a | no |
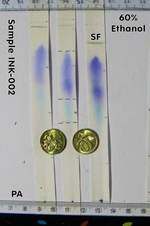
| 3 * | Ethanol 60% | 3 | B | upper: Violet middle: Light Blue lower: Grey streak from PA |
YES |
| 4 | Ethanol 70% | 2 (3) | B | upper: Violet middle: Light Blue lower: Grey streak from PA |
no |
| 5 * | Methanoic Acid 75% | 3 | B | upper: Violet, comet shaped middle: Light Blue lower: Black/grey streak from PA |
YES |
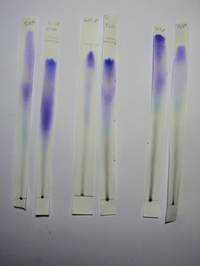
| 6a * | Propanone 80%, 70% | 3 (5?) | B | upper: Intense violet zone which in 70% formed 2 intense loci (= 2 violet dyes) middle: Very faint yellowish zone followed by pale and dispersed violet zone and this followed by a Light Blue zone lower: Black/grey streak from PA |
YES |
| 6b * | Propanone 60% | 3 (5?) | B | upper: Intense violet zone middle: Very faint yellowish zone followed by pale and dispersed violet zone and this followed by a Light Blue zone all intersecting into and a continuous streak. No true separation lower: Black/grey streak from PA |
YES |
| 6c * | Propanone 50% | 5 | B | upper: Pale violet zone middle: Dirty yellow zone followed by an intense violet zone and below a light blue zone lower: Black/grey streak from PA |
YES |
| 8 * | Chloroform | None | U | n/a | YES |
| 9 * | Kerosene | None | X | Main ink did not dissolve, but a faint greyish zone moved half way up the paper strip. | Yes |
| 10 | Chloroform/Kerosene Mixture 1:1 | 2 | U, B | A yellow / pale brown zone was separated above the main colourful dyes which remained as a streak from the PA | Yes |
| 11 | Formaldehyde | None | X | n/a | no |
| 12 | Xylene | None | U | n/a | no |
| 13 | DiMethylSulphOxide (50%) | None | U | n/a | no |
| 14 | DiMethylSulphOxide (100%) | None | U | n/a | no |
| 15 * | Propanone/Ammonia mixture (7:3) | 4 | U, O | upper: Compact violet dye followed by and intersecting with a bluish-violet dye middle: Light blue isolated zone about 2cm below the upper violet zone lower: Greyish black streak from PA which fades away upwards. |
Yes |
| 16* | Propanone / Ethanol / Water mixture (1:1:2) | 3 | O, U | upper: Compact violet zone very close to solvent front middle: Oval violet zone well separated from the zone above, followed by a weak light blue zone lower: Greyish black streak from PA which fades away upwards |
no |
| 17 * | Propanone / Ethanol / Water mixture (3:3:4) | 4 | O, U | upper: Compact violet zone very close to solvent front middle: Very faint yellow zone followed by a Violet zone (elongated shape) with a light blue zone just below and intersecting with it lower: Greyish black streak from PA which fades away upwards, |
Yes |
 |
 |
 |
 |
| Propanone 50% - 70% | Ethanol 60% | Chloroform:Kerosene 1:1 | Propanone:Ethanol:Water 1:1:2 |
[ Main Page ] - [ Methodology ] - [ Solvents Used ] - [ Rf Values ] - [ Ink Sample Analysis ] - [ Extracted Dyes ] - [ History ]
Chemistry Section Links[ Chemistry Main Page ] - [ Qualitative & Quantitative Analysis ] - [ Pyrotechnics ] [ Cation ID ] - [ Chromatography ] - [ Crystal Study ] - [ Misc Documents ] - [ Chemistry Index Page ]