CHEMISTRY :
Chromatography
Choice of Solvents and their properties
| PROJECT CODE: |
4.01a |
| SECTION: |
CHROMATOGRAPHY |
| PROJECT TITLE: |
Solvents and their characteristics |
| RELEASE DATE: |
02 Jan 99 |
| LAST UPDATE: |
1 Sep 2009 |
| VERSION HISTORY: |
1.0 - First release
1.1 - ? 9 Jan 1999
2.0 - ? 30 Jan 1999
2.1 - ? 7 Feb 1999
2.2 - Revision of text and formatting. (2-Sep-2009)
|
INTRODUCTION:
Different dyes, pigments and solutes move best along the chromatography paper with some solvents and worst with others. In other words every solute have a specific Rf value fore every solvent, and even for different concentrations of the same solvent in water or other miscible solvents. For example some pigments have a better Rf value (= moves further away rom the application point) in 60% Ethanol than in absolute (98%) Ethanol. In fact solvents can be used as follows :
- Pure single solvent (100% Conc.)
- A solvent at a certain diluted concentration in water
- A mixture of 2 or more miscible solvents at a certain ratio
The ideal solvent would be that which seperates the components of the test material into seperate concentrated zones at a good distance away from each other, each made up of only one of the consituents present as illustrated in the diagram below.
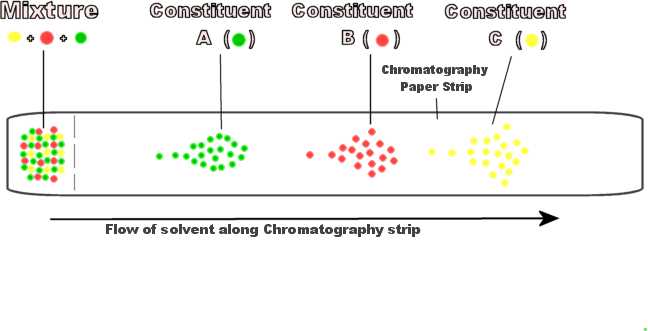
The following list of solvents below were used to study the chromatography action on pen ink, (an ordinary ballpoint pen) using gradual concentrations of solvent where possible.
- METHANOL - (variable concentrations)
- ETHANOL - (variable concentrations)
- BUTANOL - (absolute)
- CARBON TETRACHLORIDE - (absolute)
- CHLOROFOEM - (absolute)
- METHANOIC ACID - (variable concentrations)
- PROPANONE - (variable concentrations)
TEST MATERIAL and EXPERIMENT CONDITIONS
All test were carried out at same room temperature, same method, and same test material, which was a standard ballpoint ink called "Corvina - 91" - (Link 2)
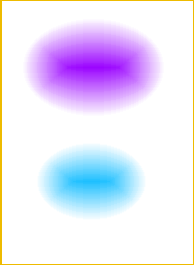
The ink was found to be composed of two dyes:
1) Violet dye - (60-65% approx.)
2) Cyan dye - (35-40% approx.)
These colours can be seen in the picture below:
The chromatography strip was 12cm long and the marked percentages were represents by 10 equal marks each 12mm apart. If the length of solvent travels from the point of application (0%) to 12cm above, it would mean 100% flow. The procedure can be read in this [dedicated webpage ] which uses the setup shown in the diagram below
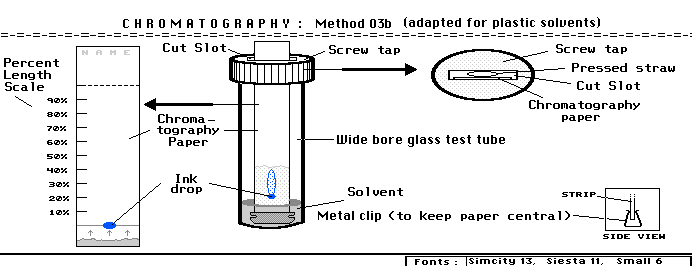
Separation OF INK COMPONENTS BY DIFFERENT SOLVENTS
MISCIBILITY CHARACTERISTICS OF SOLVENTS
Some solvents cannot be used at variable concentrations since they are not
miscible with water. The following table demostrates the solubility of solvents with each other.
Solvent Miscibility Table
| SOLVENT NAME |
WATER |
METHANOL |
ETHANOL |
BUTANOL |
CARBON TETRA CHLORIDE |
CHLORO- FORM |
METHANOIC ACID |
PROPANONE |
| WATER |
X |
Y |
Y |
N.1 |
N.2 |
N.2 |
Y |
Y |
| METHANOL |
Y |
X |
Y |
Y |
Y |
Y |
R |
Y |
| ETHANOL |
Y |
Y |
X |
Y |
Y |
Y |
R |
Y |
| BUTANOL |
N.1 |
Y |
Y |
X |
Y |
Y |
R |
Y |
| CARBON TETRA CHLORIDE |
N.2 |
Y |
Y |
Y |
X |
Y |
N |
Y |
| CHLORO- FORM |
N.2 |
Y |
Y |
Y |
Y |
X |
N |
Y |
| METHANOIC ACID |
Y |
R |
R |
R |
Y |
Y |
X |
Y |
| PROPANONE |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
X |
Legend:
- X = Not applicable (same solvent )
- Y = YES - it is miscible forming a homogenous solution
- N = NO - it is not miscible forming two seperate layers
- N.1 = NO - Forms a cloudy emulsification solution on shaking which on settling for about an hour 2 separate layers are formed, of which the top layer is butanol, and the other is the alcohol
- N.2 = NO -Forms immediately two layers, of which water forms the upper layers.
- R = REACTS - A reaction between the two liquids takes place such as an Esterification reaction between an alcohol and a carboxylic acid.
FINAL NOTES
Chloroform, Tetrachloromethane and Butanol, cannot be
diluted with water to form variable concentrations.
If water, an alcohol and chloroform (or tetrachloromethane) are mixed together,
an upper layer of water and a bottom layer of alcohol + chloroform is formed.
This shows that alcohol prefers to dissolve in chloroform rather than in water.
The following are solvents which can dissolve plastics, hence the chromatography
of these must be performed in glass containers/test tubes.
Tetrachloromethane (Carbon tetrachloride) C.Cl4
Trichloromethane (Chloroform) CH.Cl3
Propanone (Acetone) CH3.CO.CH3
Chromatography Section Links :
[ Main Page ] -
[ Methodology ] -
[ Solvents Used ] -
[ Rf Values ] -
[ Ink Sample Analysis ] -
[ Extracted Dyes ] -
[ History ]
Chemistry Section Links
[ Chemistry Main Page ] -
[ Qualitative & Quantitative Analysis ] -
[ Pyrotechnics ]
[ Cation ID ] -
[ Chromatography ] -
[ Crystal Study ] -
[ Misc Documents ] -
[ Chemistry Index Page ]




